Enzymatic Browning in Fruits & Vegetables

We all might have experienced the browning of apple or potato when their cut pieces are kept for some time in the open air. This is the best and common practical example of enzymatic browning in fruits and vegetables. In this article, we will talk about the science of this enzymic browning.
Phenolase browning, commonly called enzymic browning is of great commercial significance, particularly in fruits and vegetables where phenolase enzymes are common.
In intact tissue, phenolic substrates are separated from phenolase and browning does not occur. Enzymic browning does not occur. Enzymic browning can be observed on the cut surfaces of light-colored fruits and vegetables, such as apple, bananas, and potatoes.
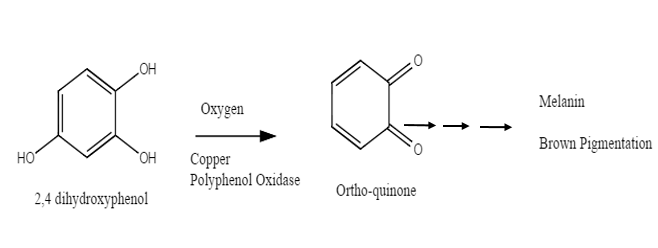
Exposure of the cut surfaces to air results in rapid browning due to the enzymatic oxidation of phenols to orthoquinones, which in turn rapidly polymerize to form brown pigments or melamines in fruits and vegetables.
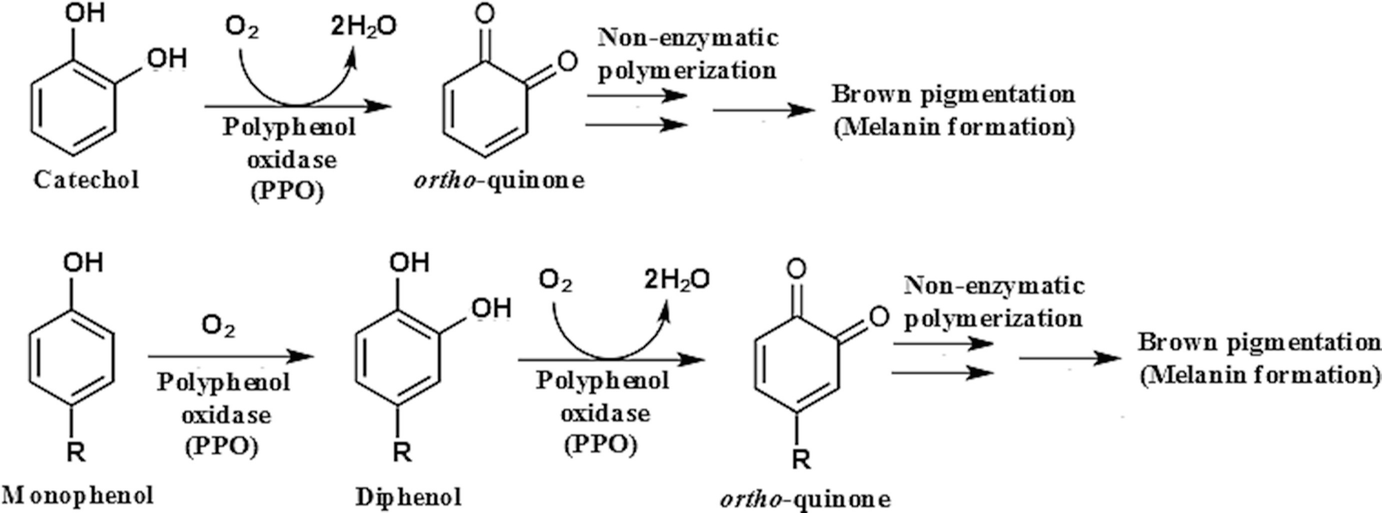
The various enzymes that catalyze oxidations of phenols are commonly known as phenolases are polyphenol oxidase (PPO), tyrosinases, or catecholases.
Phenolase catalyzes two types of reactions – hydroxylation A (referred to as phenol hydroxylase or cresolase activity) which results into results in orthohydroxylation of a phenol and oxidation B (referred to as polyphenol oxidase or catecholase activity) which results into in oxidation of the diphenol to an orthoquinone.
If tyrosine in the substrate, phenolase catalyzes its hydroxylation to DOPA, and subsequently catalyzes oxidation of DOPA to DOPA quinone. The remaining portions of the reaction sequence involve non-enzymic oxidations and ultimately polymerization of indole 5,6-quinone to brown melanin pigments. Furthermore, the melanins can interact with proteins to form complexes.
Method to control enzymatic browning
1. pH control
In general, phenolase is active between pH 5 and 7 and does not have a very sharp pH optimum. At lower pH values of approximately 3 the enzyme is irreversibly inactivate
2. Exclusion of oxygen
This is the most common method of controlling enzymic browning. One general practice is dipping potato slices in water before frying to avoid enzymic browning of potato.
3. Use of sulphur dioxide or sulphite
This is method is useful for the product which can undergo textural or flavour changes upon heating. It is the powerful inhibitors of polyphenol oxidase.
Treatment is either in the form of gaseous sulphur dioxide or as a dilute solution of sulphites. Its mode of action is in two ways. First changes in the protein conformation of polyphenol oxidase. Secondly, it forms a colourless complex with quinone thus preventing the polymerization into melanin
4. Application of heat
Application of heat to the food article at a high temperature for an adequate length of time inactivates phenolase and all other enzymes present.
Heat applied such as blanching and high temperature- short time (HTST) pasteurization used respectively in the pre-treatment of vegetables for canning, freezing preservation or dehydration, or in the manufacture of fruits juice and purees.
5. Exclusion of oxygen
The simplest application of this method of phenolase inhibition is often found in the home at a time when it is convenient to peel and cut potatoes sometime before cooking.
During the intervening time interval, they are submerged in water, thus limiting the oxygen which can reach the cut potato tissue
6. Inhibition by Sodium Chloride
This method of inhibition of phenolase has had comparatively limited use being employed mainly for holding peeled fruits of processing. A model system demonstrated that 0.1% sodium chloride significantly inhibited browning.
The reaction mixtures contained 0.4% chlorogenic acid buffered at pH 5.0, water or sodium chloride solution and preparation of phenolase from apples.
7. Methylation of phenolase substrates
The fact that substances such as guaiacol and ferulic acid will not function as phenolase substrates have already been mentioned. A method for preventing enzymic browning in F&V has been developed based on the enzymic methylation of the o-methyltransferase.
o-methyltransferase is believed to be involved in the biosynthesis of lignin and other aromatic compounds. The enzymes will function in the presence of a methyl donor such as S-adenosylmethionine and a methyl acceptor such as an o-diphenol.
8. Application of acids
This is a widely used method for the control of enzymic browning. The acids employed are among those which occur naturally in tissues, particularly citric, malic, phosphoric, and ascorbic acids. In general, their action is to lower the tissue pH and thus to decrease the rate of browning.
9. Boric acid and borat
Browning was completely inhibited by treatment with 1.5% sodium tetraborate and with 1.5% sodium metaborate.
You may be interested in



Very valuable topic sir. Its really works alot.
Thanks for the appreciation
Thank you for this very thorough explanation of enzymatic browning and the factors that can cause (and therefore prevent) it. On a commercial scale, fresh produce processors have found that not all food acids are equal. Some impart a very sour flavor (think lemon juice or vinegar or citric acid), but are effective at lowering the pH enough to retard the enzymatic browning process. Other acids don’t make such a sour taste and are very effective. Antioxidants, such as sulfites, also work well, but do not have the same level of consumer acceptance (some people are sensitive to sulfites).
https://tlcingredients.com/sodium-acid-sulfate/